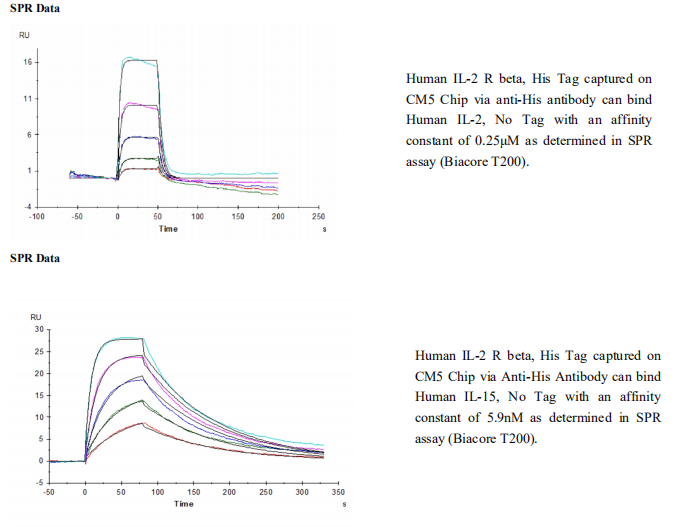
Functional IL-2 receptors can exist in two affinity states on cell surfaces, the high affinity complex consisting of heterotrimers of the alpha, beta, and gamma chains, and the intermediate affinity complex comprising heterodimers of the beta and gamma chains. Individual beta chains and alpha chains exhibit low affinity IL-2 binding and the gamma chain alone does not bind IL-2. In addition to their involvement in IL-2 mediated signal transduction, both the beta chain and gamma chain have been shown to be required for IL-15 mediated signaling. IL-2 R beta is a member of the cytokine receptor superfamily. Human IL-2 R beta cDNA encodes a 551 amino acid residue precursor Type I membrane protein with a 26 residue signal peptide, a 214 residue extracellular region, a 25 residue transmembrane region and a 286 residue cytoplasmic domain. A soluble IL-2 R beta (IL-2 sR beta ) has been identified in the culture supernatants of a human lymphoid cell line, YT, that displays IL-2 R beta. At present, the function of IL-2 sR beta is unclear. Recombinant human IL-2 sR beta binds IL-2 with low affinity and is not an effective IL-2 antagonist on cells displaying the high or intermediate affinity IL-2 signaling receptors. Nevertheless, IL-2 sR beta binds IL-15 with sufficient affinity to neutralize IL-15 biological activities.
高纯度、高活性、低内毒素、高批间一致性
产品数据
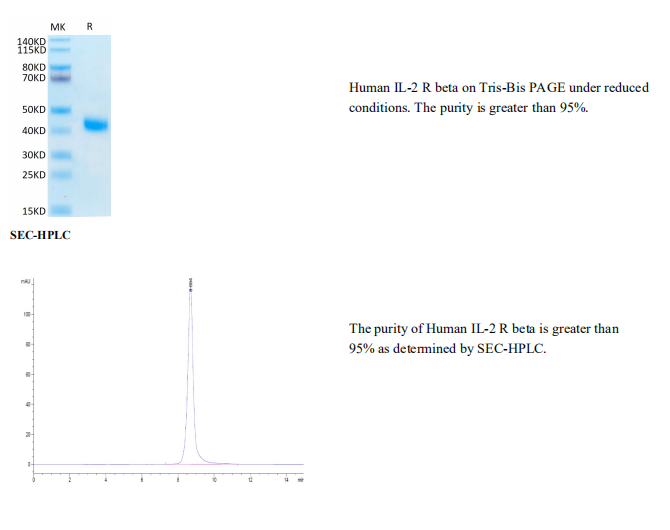
Tris-Bis PAGE
-25 ~ -15℃保存,收到货之后有效期1年。 复溶后, 无菌条件下,-85 ~ -65℃保存,3个月有效期。

